Previous Winners
With the yearly Pfizer Oncology Award, Pfizer wants to support the care for cancer patients and reward projects that improve the quality and/or availability of patient care in oncology and haemato-oncology. The award is intended to support, recognise and encourage innovative projects in Belgium or Luxemburg. The Pfizer Oncology Award is open to any project related to the clinical, surgical, medical or technical aspects of patient care in oncology, but also to different ways of collaboration and/or organisation between various healthcare providers or between levels of care during the course of the disease. The winning project is selected by an esteemed panel of medical oncologists, haematologists, and onconurse representatives, chaired by Prof. Sylvie Rottey (University Hospital Ghent). During the 2024 annual BSMO-Bordet symposium on the Integration of Molecular Biology Advances into Oncology Clinical practice, the 9th Pfizer Oncology award of 25.000 € was granted to Dr. Eline Naert, University Hospital Ghent, for setting up the HAIR study.

HAIR study: hair-loss alleviation by using minoxidil in patients with metastatic breast cancer treated with endocrine therapy in combination with a CDK4/6 inhibitor
Eline Naert, Medical Oncologist, University Hospital Ghent.
Alopecia induced by endocrine therapies (ET) is a widely recognised adverse event that negatively impacts quality of life (QoL) of breast cancer patients.1,2 Individuals experiencing cancer treatment-induced alopecia are 1.5 times more prone to depression compared to those with lower distress levels. In clinical trials, alopecia is classified as grade 1, defined as hair loss <50% that is only obvious on close inspection, and grade 2, defined as hair loss ≥50% that is apparent to others and warrants a wig or hair piece if the patient desires to completely camouflage the hair loss.3
Currently, international guidelines recommend the addition of a cyclin dependent kinase 4/6 inhibitor (CDK4/6i; abemaciclib, palbociclib or ribociclib) to ET for patients with hormone receptor-positive (HR+) human epidermal growth factor receptor 2 negative (HER2-) metastatic breast cancer based on the outcomes of multiple randomised phase III trials showing improved survival outcomes compared to ET alone. However, CDK4/6i work by inducing cell-cycle arrest, which can further contribute to hair growth impairment and increase the incidence of alopecia.1,2 Indeed, cumulative data indicate a composite all-grade alopecia incidence of 15-30% with a combination of ET and CDK 4/6i, as compared to 9.6% for patients receiving exclusively ET.
In addition, patients receiving CDK4/6i have prolonged treatment durations. Although hair loss is not life threatening and not dose limiting, it has a psychological and social impact and might be a reason for treatment discontinuation.4,5 Non-surprisingly, the concerns on alopecia always comes along with the patient’s request for therapy to counterbalance the hair loss. However, an effective remedy for hair loss in this specific setting has not yet been studied, rendering it unknown. As such, there are many patients that have expressed their frustration due to the lack of attention, knowledge, and research to address this need. The HAIR trial therefore investigates the use of minoxidil as a potential strategy to help these patients.
Study design
The HAIR trial is a longitudinal, single-arm, multi-centre, Belgian study that will evaluate the administration of topical minoxidil 5% (2x/d 1 ml on the dry scalp) for 9 months in 200 patients with metastatic breast cancer currently treated with CDK4/6i and ET for at least 3 months. This evaluation will be done quantitatively and qualitatively. The quantitative data collection will be obtained by patient-reported outcome (PRO) measurements and clinician-reported outcome (ClinRO) measurements at baseline, after 4.5 months and after 9 months of minoxidil administration and 3 months after completing minoxidil treatment. After 9 months of minoxidil treatment, the treating physician will discuss continuation (outside the HAIR study) versus discontinuation of minoxidil with the patient. PRO questionnaires will investigate the psychosocial impact of alopecia (CADS, chemotherapy-induced alopecia distress scale), the quantification of hair loss (PRO-CTCAE question 27), the usability of minoxidil (TPUQ, topical product usability questionnaire), and health-related QoL (EORTC QLQ-C30). The ClinRO measurements will evaluate the effect of minoxidil on hair growth (Sinclair scale). Qualitative data will be collected by semi-structured one-on-one interviews in a subset of patients with the aim of identifying the impact of hair loss and of giving insights regarding usability of topical minoxidil 5%. Patients for this study will be recruited in University Hospital Ghent, AZ Groeninge Kortrijk, AZ Delta Roeselare, Jan Yperman Ieper, AZ Sint Jan Bruges, and ASZ Aalst.
If the study results are positive, this will result in a significant impact and will potentially benefit thousands of breast cancer patients treated with CDK4/6i and ET worldwide. Topical minoxidil is widely available and affordable in most countries. Therefore, clinical implementation might occur quickly after dissemination of results.
References
- Silvestri M, et al. Drug Safety. 2021;44:725-32.
- Eiger D, et al. Acta Oncologica. 2020;59(6):723-25.
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Accessible through https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf
- Freites-Martinez A, et al. JAMA Dermatol. 2018;154(6):670-5.
- Moscetti L, et al. Tumori. 2015;101(5):469-73.
With the yearly Pfizer Oncology Award, Pfizer wants to support the care for cancer patients by rewarding projects that improve the quality and/or availability of patient care in oncology. In 2023, the “classical” Oncology Award of 20.000€, awarded to a project focusing on patient care in oncology and/or hematology, was complemented with a new Oncology/Covid Award of 10.000€, rewarding a project that focuses on the care of oncology and/or hematology patients in the context of Covid-19. During the 2023 annual BSMO-Bordet symposium on the Integration of Molecular Biology Advances into Oncology Clinical practice, the 8th Pfizer Oncology Award was granted to Prof. Dr. Melissa De Regge (University Hospital Ghent), while Ms. Yana Debie and Prof. Dr. Timon Vandamme (University Hospital Antwerp) received the 1st Pfizer Oncology/Covid Award. 
Improving the scan-journey: co-creation with patients and their informal caregivers to reduce Scanxiety
Prof. Dr. Melissa de Regge (University Hospital Ghent)
Scanxiety describes the anxiety patients experience when anticipating upcoming scans and while they wait for their results. Scanxiety is common among cancer patients, and it is recognized to have a detrimental impact on their quality of life. Nevertheless, important research gaps exist around scanxiety. First and foremost, there is a lack of standardized tools to measure scanxiety. In addition to this, little is known on how scanxiety evolves over time, nor have researchers extensively investigated interventions to reduce scanxiety. With their winning project, the research team of Prof. De Regge wants to measure the prevalence and severity of scanxiety at different timepoints along the scan-journey, explore which factors have an influence on the experience of scanxiety in patients and caregivers and design interventions to reduce scanxiety in co-creation with patients, informal caregivers, and care providers.
In the first part of this project, a widely applicable instrument was developed to assess scanxiety at different timepoints during the scan journey. This questionnaire was pilot tested by care providers, experts in service management, a patient advisory board and patients. To validate the questionnaire, a field test was subsequently performed in four patient groups (melanoma, breast cancer, lymphoma, and myeloma). In the second phase of the study, this questionnaire will be used to assess how scanxiety plays a role at different timepoints along the scan-journey and to gain insights into the variables that influence scanxiety (e.g., fear of progression, fear of the scan procedure, stress associated with traveling to or being in the hospital, etc.). The goal of the final phase is to develop interventions that can reduce anxiety. These interventions will be developed using service design thinking method adopting co-creation workshops with patients, informal caregivers and care providers involved in the oncological care pathway (onconurses, oncologists, onco-psychologists, general practitioners, etc.). The choice for such a design will assure that the needs of all stakeholders will be met. The target of the project is to develop different co-created interventions that are ready for implementation, with the goal to reduce scanxiety for patients and their informal caregivers and ultimately enhance their quality of life.
Cellular and humoral immune responses after covid-19 vaccination in cancer patients (CLOVER)
Yana Debie & Prof. Dr Timon Vandamme (University Hospital Antwerp)
Although many studies have been conducted evaluating the immunological responses after Covid-19 vaccination, parts of the vaccination-induced immune response remain understudied. The primary objective of the research project presented by Ms Yana Debie is to unravel whether natural killer (NK) cell signatures can predict a humoral immune response to Covid-19 vaccination in cancer patients. To this end, the project will use samples of cancer patients who received 2 doses of the BNT162b2 or ChAdOx1 vaccines and up to 2 booster doses of BNT162b2 over a period of 14 months. Baseline NK-cell signatures of these patients will be generated after which the investigators will look for associations between these signatures and the establishment of vaccination-induced antibodies.
NK-cell analyses will be performed on cryopreserved peripheral mononuclear cell samples that were taken prior to vaccination. The NK-cells in these samples will be analysed using flowcytometry after which specific software (FlowJoTM software package) will be used to generate NK-cell signatures. Subsequently, these NK-signatures will be correlated with the anti-S1 SARS-CoV-2 IgG responses 28 days after the second vaccination dose to reveal whether the NK-signature at baseline has a predictive capacity for the outcome of vaccination-induced humoral immunity. The information that will be obtained through this process will then be used to build a model that can be used in clinical practice to identify patients who will not respond to Covid-19 vaccination. This will avoid needless vaccination in patients who are unlikely to respond, safeguarding them from potential side effects and allows to intensify vaccination and other protective measures in the patient’s environment.
Exceptionally in 2022, Pfizer rewarded two innovative projects improving the quality and/or availability of patient care in oncology and haemato-oncology. In fact, during the 2022 annual BSMO-Bordet symposium on the Integration of Molecular Biology Advances into Oncology Clinical practice, the 7th Pfizer Oncology award was granted to both Tiene Bauters (PharmD, PhD), University Hospital Ghent and Laura Elst (MD), University Hospitals Leuven.

Understanding and mitigating risks that can compromise the quality and safety of anticancer biotherapeutics due to pneumatic tube transport in hospitals.
Tiene Bauters, PharmD, PhD, University Hospital Ghent
The use of biotherapeutics, such as monoclonal antibodies, has increased substantially over the last decade. In contrast to small molecule drugs, these biotherapeutics have a very complex and sensitive structure. Pharmaceutical companies make an enormous effort to deliver high-quality and safe biotherapeutics to hospital pharmacies. However, after the delivery, these biotherapeutic agents undergo additional handling steps (e.g., reconstitution, dilution, mixing, transport to the patient, administration) often summarised under the term “the last mile”. The steps during “the last mile” are not controlled by the drug manufacturer and the handling practices can differ substantially across different healthcare institutions. Therefore, “the last mile” represents a significant and poorly understood gap in the process of providing high-quality and safe biotherapeutics to patients. One of the major risk factors during this process is mechanical stress during drug transport from the hospital pharmacy to the patient. Especially the widely used transportation with pneumatic tube systems (PTS) was recently identified as a potential cause of biotherapeutic degradation. The mechanical stress during transport can cause protein aggregation, which is a major degradation pathway of biotherapeutics. Such protein particles increase the risk of side effects, including severe allergic responses or the generation of anti-drug antibodies, and can lead to treatment discontinuation.
The winning project by Tiene Bauters and colleagues aims to evaluate whether pneumatic tube transport of frequently prescribed anticancer biotherapeutics (nivolumab, pembrolizumab, bevacizumab, pertuzumab, and rituximab) from the hospital pharmacy to the oncology ward causes protein aggregation, which can compromise drug safety and quality. To this end, samples will be taken before and after PTS transport. Five to ten replicates obtained on different days will be included for each drug. These samples will then be analysed by a set of complementary techniques used to cover an aggregate size range from nm to mm. Secondly, investigators want to determine how variables such as transport distance, soft vs. hard tube landing, temperature and packaging material affect the aggregation of anticancer biotherapeutics during pneumatic tube transport in the hospital. The final objective is to draft guidelines and establish best practices for in-hospital transport of anticancer biotherapeutics. These guidelines will reduce the risk of accidental transport induced aggregate and particle formation during in-hospital transport, ultimately reducing the risk of aggregate-associated adverse events in cancer patients.
Implementation of interactive stratification tools for penile cancer patients to determine their individual need for patient education or psychosexual counselling.
Laura Elst (MD), University Hospitals Leuven
Since 2015, a penile cancer care centralisation pathway has been implemented in the University Hospitals Leuven. This implementation resulted in an optimisation of care pathways, an increased experience of clinicians, a higher use of organ-sparing surgery and less invasive lymph node staging techniques, which results in a better patients’ quality-of-life (QoL). Nevertheless, despite this care centralisation, the diagnosis and surgical treatment of penile cancer remains to have a major impact on voiding function, sexuality, psychological well-being and QoL. The difficulties experienced by patients vary, but include erection problems, problems with sexual intercourse, confrontation with a different look of the penis during daily care, and anxiety hurting or damaging the penis. On top of that, the associated taboo and ignorance of penile cancer makes it harder to seek support in the social environment. Moreover, embarrassment may hamper patients to ask for professional help. Unfortunately, during a consultation with the urologist there is often a lack of time to fully address all these psychosexual needs and to date, there is a lack of information brochures, videos or specialised nurse consultations that provide relevant information for patients.
With her award-winning project, Laura Elst aims to improve the psychosexual and functional adaptation to penile cancer by providing information brochures and patient videos on the potential outcomes of penile cancer therapies. In a first phase, a script will be created with the necessary themes that have to be addressed in the brochure and the educational videos. After validation of this script by the different stakeholders and a patient council, the brochures and patient videos will be produced. Additionally, this information will be made more attractive with visual materials, experiences from patients and quotes from partners. The urologist will discuss the information and hand over the materials, which could help the patients and their partners to grasp the multitude of information and to discuss the impact on sexuality. Furthermore, the study group wants to create a pre-treatment questionnaire which assesses the individual need for psychosexual counselling. With this assessment, possible concerns can be picked up quicker, facilitating a more rapid and easier referral.
Finally, the satisfaction before and after implementation of these education and stratification tools will be evaluated. If the project proves to be successful, the information tools can be translated into French and English and the information can be made available on the internet, so that also other penile cancer patients can benefit.
Preventing emotional dysregulation in metastatic breast cancer patients

During the 15th BSMO-Bordet symposium on the Integration of Molecular Biology Advances into Oncology Clinical practice, Prof. Isabelle Merckaert and Dr. Florence Lewis (Institute Jules Bordet, Brussels, Belgium) were awarded with the 6th Pfizer Oncology Award. This yearly award, worth 25,000 Euro, is intended to support, recognise, and encourage innovative projects in Belgium or Luxembourg that improve the quality and/or availability of patient care in oncology. The Pfizer Oncology Award is open to any project that addresses the clinical, surgical, medical, or technical aspects of patient care in oncology. In addition, also projects focussing on different ways of collaboration between various healthcare providers or different levels of care during the cancer journey are invited to contend for the award. The winning project is selected by an esteemed panel of medical oncologists, haematologists, and onconurse representatives, chaired by Prof. Sylvie Rottey (University Hospital Ghent). The winning project of the 2021 Pfizer oncology award aims to compare the impact of a high-intensity group intervention on the prevention of emotional dysregulation in metastatic breast cancer patients to that of a moderate-intensity group intervention.
Psychological interventions to fight emotion dysregulation in metastatic breast cancer patients
Patients with metastatic breast cancer face a wide range of challenges. Importantly, these challenges are not only physical as the disease also puts a heavy emotional, social, and spiritual burden on patients. As a result, patients are often confronted with intense negative emotions throughout their disease course. While these emotions can help to adapt and cope with the disease, excessive negative emotions can lead to emotional dysregulation, which in turn has a detrimental effect on patients’ quality of life and can lead to mood disorders. To ameliorate these complications, current guidelines recommend early psychological interventions for patients with metastatic breast cancer. Although different types of interventions have shown to be effective, there is still no consensus on the most effective content, format, and intensity of these interventions to prevent emotional dysregulation in patients. A previous study carried out at Institute Jules Bordet demonstrated the effectiveness of an early intervention, using questionnaires and ecological (EMA) and dynamic assessment methods on emotional regulation. This study targeted breast cancer survivors in the early survivorship. However, the efficacy of an early intervention has not been yet properly analysed in the metastatic setting.
The winning project wants to enable metastatic breast cancer patients to acquire skills and offer tools to prevent emotional dysregulation in their daily life. The primary objective of the study is to compare the impact of two different interventions: High-Intensity Emotion and Self-Regulation Group Intervention (HIGI) and Moderate-Intensity Emotion and Self-Regulation Group Intervention (MIGI). The aim of this project is to compare two interventions on both the dose (i.e., number of sessions) and intensity (i.e., frequency of sessions) needed to address patients’ needs and difficulties. The efficacy of these interventions is analysed just after the intervention (immediate efficacy) and five months thereafter (mid-term efficacy). The study will also compare the effect of these interventions on global adjustment, and intervention benefits.
Study design
The study at hand will include patients newly diagnosed with metastatic breast cancer or distant recurrence treated in the Institute Jules Bordet in Belgium. A pre-assessment screening will be conducted after the first oncological check-up, after which patients with less than one year of life expectancy will be excluded. Other exclusion criteria include severe cognitive impairment and current severe and/ or acute psychiatric disorders. Subsequently, participating patients will be randomly assigned to HIGI or MIGI. The study aims to include a total of 113 patients.
The interventions are built up of three elements: group sessions, ecological booster sessions and an e-platform. The group sessions will integrate an emotion regulation, a psycho- educational, an existential and a cognitive behaviour components. These sessions will be enhanced by tailored ecologically booster sessions, which aim to promote the practice of the techniques learned during the sessions. Between sessions, patients will also receive further instructions from a smartphone application. These app-based prompts are organized into modules that are sent to the patients each day. In addition to this, an e-platform gives access to the tools and techniques learned during the different sessions. This platform includes psycho-educational video clips addressing among other things different emotions, a question list to support communication and hypnosis recordings in order to reinforce empowerment.
The MIGI group intervention will consist of eight 2.5-hour group sessions every two weeks. This intervention will aim to provide patients with basic tools to help them cope with the chronic disease. The HIGI will aim to provide patients with the same basic tools as well as to reinforce the acquisition of these tools and the transfer of them into everyday life through six additional sessions. This intervention will thus consist of fourteen 2.5-hour group sessions every week.
The results will be analysed at the beginning of the treatment and after 5 and 10 months. The primary outcome will be the general and specific emotion regulation. This will be measured in three ways. First, the dynamic emotion regulation task involves exposure to cancer-related triggers by completing self-report questionnaires and relaxation exercises. Secondly, the Ecological Momentary Assessment (EMA) provides a picture of patients’ emotion regulation in their everyday life thanks to an armband and a smartphone application. Finally, emotion regulation will be analysed by different questionnaires. In addition to this, other outcomes, such as global adjustment and intervention benefits will also be assessed.
The goal is to start recruiting patients in February 2022, followed by assessments and interventions. The statistical analysis and writing of the articles will begin in March 2022 and finalize in January 2025. The interventions and evaluations of the project will be financed by the Centre of Psycho- Oncology and the Clinique of Psycho-oncology of the Institute Jules Bordet. The 25,000 euros associated with the Pfizer oncology award will allow the creation of the e-platform and the psycho-educational videos clips offered to the study participants. After the end of the study, the aim is to make these video clips available to as many patients as possible.
The use of emotional freedom techniques to reduce fear of cancer recurrence

Dr. Philip Debruyne (AZ Groeninge) has been awarded with the 2020 Pfizer Oncology Award for his project which aims to examine the efficacy of Emotional Freedom Techniques (EFT) to reduce the fear of cancer recurrence.
Emotional freedom techniques and fear of cancer recurrence
Although there are over a quarter of a million cancer survivors in Belgium, the mental wellbeing and quality of life of patients after their cancer diagnosis and treatment is often poorly addressed. As a result, many cancer survivors live with a fear of cancer recurrence (FCR) and approximately one third of cancer survivors indicate they would need professional help to cope with the fear, worry or concern that their cancer may return or progress. Although cognitive behaviour therapy, mindfulness and yoga might be valuable in relieving stress, anxiety and depression in these patients, these techniques usually require an intensive program. Emotional freedom techniques (EFT) is an easy to learn self-help tool that might be a suitable alternative. EFT was originally developed to manage anxieties and phobias and is comprised of a somatic and a cognitive component. The somatic component includes acupressure (specific points on the head, face, collarbone and under the arm) while the cognitive component refers to a psychological tool in which one addresses a problem, complaint, worry or concern out loud. With these techniques, a conditioned response helps to induce tolerance to the stimulus that causes anxiety. Dr. Debruyne further explains: “The trial will be conducted on behalf of the BSMO Cancer Survivorship Task Force and builds on our previous research trial examining the effect of EFT as an intervention strategy to reduce patient-reported cognitive complaints in cancer survivors (EMOTICON: NCT02771028). In this trial, we observed positive effects of EFT on secondary endpoints such as psychological distress and emotional wellbeing. As FCR is a major problem in cancer survivors and there is no widely implemented evidence-based strategy to address FCR, it is definitely worthwhile to try these emotional freedom techniques now also for this group of patients.”
Study design
Before participation, cancer patients who were previously treated with surgery, chemotherapy, radiotherapy, anti-hormonal or targeted therapy, need to complete the Cancer Worry Scale (CWS). In total, eight items are rated on a 4-point scale ranging from 1 (never) to 4 (almost always) with lower scores indicating less FCR. When scoring 14 or more, patients are diagnosed with FCR and can participate to the trial. Subsequently, they will be randomised to either the intervention or wait-list control group. In total, 43 patients in each study cohort are required. Patients in the intervention group take off with 8 weeks of practicing EFT, at least once a day, while the wait-list control group will only start their 8 weeks of EFT practice, after a waiting period of 8 weeks. Importantly, the trial is designed as a remote multicentre randomised trial and EFT training will thus be offered by an internet-based approach that allows the investigators to perform the trial with one instructor/coordinator only. This instructor is formally trained and acquired the AAMET Emotional Freedom Techniques level 1 certificate. Patients can make use of remotely organised personal contact sessions with the instructor as well as background information and instruction videos made available on an online EFT platform. Two sessions are foreseen to introduce and follow-up on the EFT practice but additional contact sessions (although virtual) with the instructor/coordinator of the trial, can be made by patients who are in need of extra support. At study entry, at week 8 (primary outcome) and at week 16, patients will be asked to complete the CWS and other questionnaires to measure the efficacy of EFT on the reduction of psychological distress, fatigue, emotional wellbeing, quality of life, health status and patients’ professional lives. “We aim to start recruiting patients in 2021 and thus far, six centres have already indicated their willingness to participate in the trial; AZ Groeninge Kortrijk, UZ Brussel (Dr. C. Fontaine), AZ Klina Brasschaat (Dr. C. Langenaeken), Jessa Ziekenhuis Hasselt (Pr. J. Mebis), AZ Glorieux Ronse (Dr. F. Vanryckegem) and UZ Gent (Pr. T. Boterberg). Nevertheless, other centres that would put themselves forward will also be offered the opportunity to participate. Depending on how fast patients will be recruited, results can be expected after roughly two years. We are, of course, very grateful to win the Pfizer Oncology Award and will spend this budget mainly on personnel costs for the responsible project coordinator for writing the protocol, obtaining approval from ethic committees, data collection and analysis, etc. The rest of the budget will be used for the development of the EFT online platform.”
Pfizer Oncology Award
During the 13th BSMO-Bordet meeting on November 22-23th 2019, professor Yves Van Nieuwenhove and Mrs. Eva Pape (University Hospital Ghent) have been awarded with the yearly Pfizer Oncology Award. This award supports, recognises and encourages one innovative project in Belgium or Luxembourg that improves the quality and/or availability of patient care in oncology and is worth 25,000 euro. Projects addressing the clinical, surgical, medical or technical aspects of patient care as well as the different ways of collaboration and organisation between various health care providers or different levels of care during the course of the disease are eligible. The winning project was selected out of nineteen submissions by an independent scientific committee, chaired by professor Sylvie Rottey and composed of medical oncologists, haematologists and onco-nurse representatives. The winning project from the group of professor Van Nieuwenhove aims at developing a LARS-clinic to improve care for rectal cancer survivors in order to manage the struggle of patients dealing with the low anterior resection syndrome .

Mrs Eva Pape (center) and prof. Yves Van Nieuwenhove receive the Pfizer Oncology Award from prof. Sylvie Rottey (Chairman of the Scientific Committee) and Pfizer delegates.
The 2018 winner of the Pfizer Oncology Award is Dr Lore Lapeire (University Hospital Ghent) for her project “The development, implementation and evaluation of a multidisciplinary oncologic consultation for adolescents and young adults with cancer in Belgium, the AYA-MOC.”
Adolescents and young adults with cancer (AYAs) struggle with finding their place within a hospital. They are too old for the paediatric department, but also the adult department fails to adequately cover all their needs. Overall, AYAs are satisfied with the medical care they receive, but this type of patients is in a phase of their lives where they have to deal with a lot of other aspects. They worry about specific subjects, like education, finding a job, sports, hobbies, sexuality, fertility, etc. All these aspects need special attention and this is often lacking in the current practice. Dr.Lapeire confirms these unmet needs from her own experience. “We still hear from patients that the risk of losing your fertility due to specific treatments has never been addressed before the start of treatment. Afterwards it is often too late.”
Due to the complexity of this patient group, Care4AYA - a multidisciplinary workgroup which aims to improve the care for AYAs - was initiated at Ghent University Hospital. So far, the workgroup has developed some helpful tools, including cards to enhance the communication between AYAs and their caregivers, and a brochure that helps parents of AYAs to better cope with their young child suffering from cancer. However, there is still a lot of room for further improvement according to dr. Lapeire. “We are in continuous dialogue with AYAs and their parents about how we can make things better for them. We also want to rejuvenate some hospital rooms to better fit the needs of AYAs. Things like internet and gaming are important in their lives, but the current hospital setting does not support this yet.”
In the upcoming year, Care4AYA will use the Pfizer Oncology Award to develop and implement an AYA multidisciplinary oncology consult (AYA-MOC). The standard MOC has a pure medical focus with specialists from different departments, but the AYA-MOC also takes into consideration physical, financial, and emotional aspects. Therefore, not only doctors, but also nurses, social workers, physical therapists, occupational therapists, and other specialists participate in the AYA-MOC. “We are still examining how we can implement the AYA-MOC in the most optimal way,” dr. Lapeire explains. “I believe that it may be especially useful in the aftercare phase, which can be difficult for patients. During their treatment, patients frequently come to the hospital and have the opportunity to talk to their caregiver more often. When they are dismissed and the number of check-ups decreases, they tend to feel lost.”
Dr. Lapeire also looks across the borders at international AYA initiatives. In France, for example, former patients don’t have to mention their previous disease state to the insurance company after a couple of disease-free years. They call it ‘the right to be forgotten’ and it increases their chances on the house and job market. Currently, the Belgian minister is trying to accomplish the same thing. In the Netherlands there are specialized reference centres, where all AYAs are discussed.Dr. Lapeire hopes to reach something similar in Belgium. “For the future it would be great if the AYA-MOC would be acknowledged and perhaps even reimbursed by the government. Then this project would be extremely successful.” At the moment, 4 hospitals in Ghent are collaborating to improve AYA care. If the AYA-MOC proves to be successful, it can be implemented in other hospitals. “It is not our goal to make all the AYAs come to our hospital,” dr. Lapeire emphasizes. “It is our goal to optimize the treatment and care of every AYA, regardless of where he or she is being treated. We are connecting all specialists that have experience with AYAs and stimulate them to share their knowledge. As such, we hope to progressively improve the AYA care together.”

Dr. Lore Lapeire (2nd from left) receives the Pfizer Oncology Award from Prof. Sylvie Rottey (Chairman of the Scientific Committee) and Pfizer delegates.
During the BSMO/Bordet meeting in Brussels, Dr. Jean-Francois Daisne (CHU-UCL Namur) was presented as the 2017 Pfizer Oncology Award winner for his project entitled: ‘Self-administration of LLLT in oropharyngeal and buccal mucositis induced by (chemo) radiotherapy of the head and neck area’.
Treatment of head and neck squamous cell carcinoma with (chemo)radiotherapy leads to severe mucositis in 25 to >50% of the patients. It is more frequent in oropharyngeal and oral cavity locations. This debilitating side effect results in pain, loss of quality of life, diet modification and subsequent weight loss and denutrition. These effects can be treated by low level laser therapy (LLLT) or photobiomodulation. This results in enhanced wound repair and tissue regeneration by influencing different phases of injury resolution including the inflammatory, proliferative, and remodelling phase. It stimulates ATP synthesis, increased tissue oxygenation and has analgesic effects. In a phase III trial it was shown that LLLT resulted in a reduction of acute mucositis compared to placebo treatment. A six-fold decrease in the incidence of grades 3–4 oral mucositis was detected in the LLLT group compared to the placebo. As a result there was also a significant reduction in pain, an increased quality of life and a reduction in parenteral nutrition use. The improvements were seen both during and after the chemoradiotherapy. LLLT did not show a detrimental effect on the chemoradiotherapy. Progression-free survival and overall survival in patients treated with LLLT was not affected in a negative way.
However, the LLLT treatment has several practical issues. The administration is performed by a radiotherapy nurse, it is time consuming and it requires the entire staff to be trained adequately. The availability of radiotherapy nurses for LLLT is limited, whereas the treatment is required daily or every other day until symptoms are resolved. To address these issues, Dr. Daisne proposed to investigate a portable device, recently developed in the Netherlands. The device consists of a small medical laser device and two laser clips that can be put in the mouth. This can be done by patients themselves in the home situation. “This is about patient empowerment”, says Dr. Daisne, “It allows them to be independent of the availability of a nurse, they don’t have to travel to the hospital to get treatment for their symptoms and they can apply it at a time of their own choice”. Dr. Daisne had the opportunity to test the device already with two patients. In both cases it resulted in pain control and limited weight loss.
In the proposed study design Dr. Daisne and his team will recruit 50 patients with painful mucositis (VAS>2). “We will be able to start around February 2018 and recruit all patients within 12 or 18 months” says Dr. Daisne. There will be a weekly recording during radiotherapy and in addition 10 and 30 days post-treatment. Data will be collected, including VAS pain scale, total time LLLT use, analgesics doses, diet consistency, parenteral nutrition and weight evolution. Quality of life will be determined using the C30 and HN35 questionnaires. Dr. Daisne: “With the support of the Pfizer Oncology Award we will be able to buy a sufficient amount of portable laser devices, and cover the costs of the data collection and insurance.” Besides the clinical results, the study will also shed light on the financial impact on healthcare costs. “Currently the use of a portable laser device is not reimbursed by health insurance, I expect however, that with this study we can demonstrate that it is feasible and cost-effective”, says Dr. Daisne. The project of dr. Daisne and his team aims to evaluate an innovative treatment modality for patients suffering from debilitating adverse events. This will help to make such innovations available nationwide and consequently improve the quality of patient care in oncology.

Dr Daisne receives the 2017 Pfizer Oncology Award from Prof Sylvie Rottey (Chairman of the Scientific Committee) and Ludwig Van den Hove (Senior Medical Manager Pfizer)
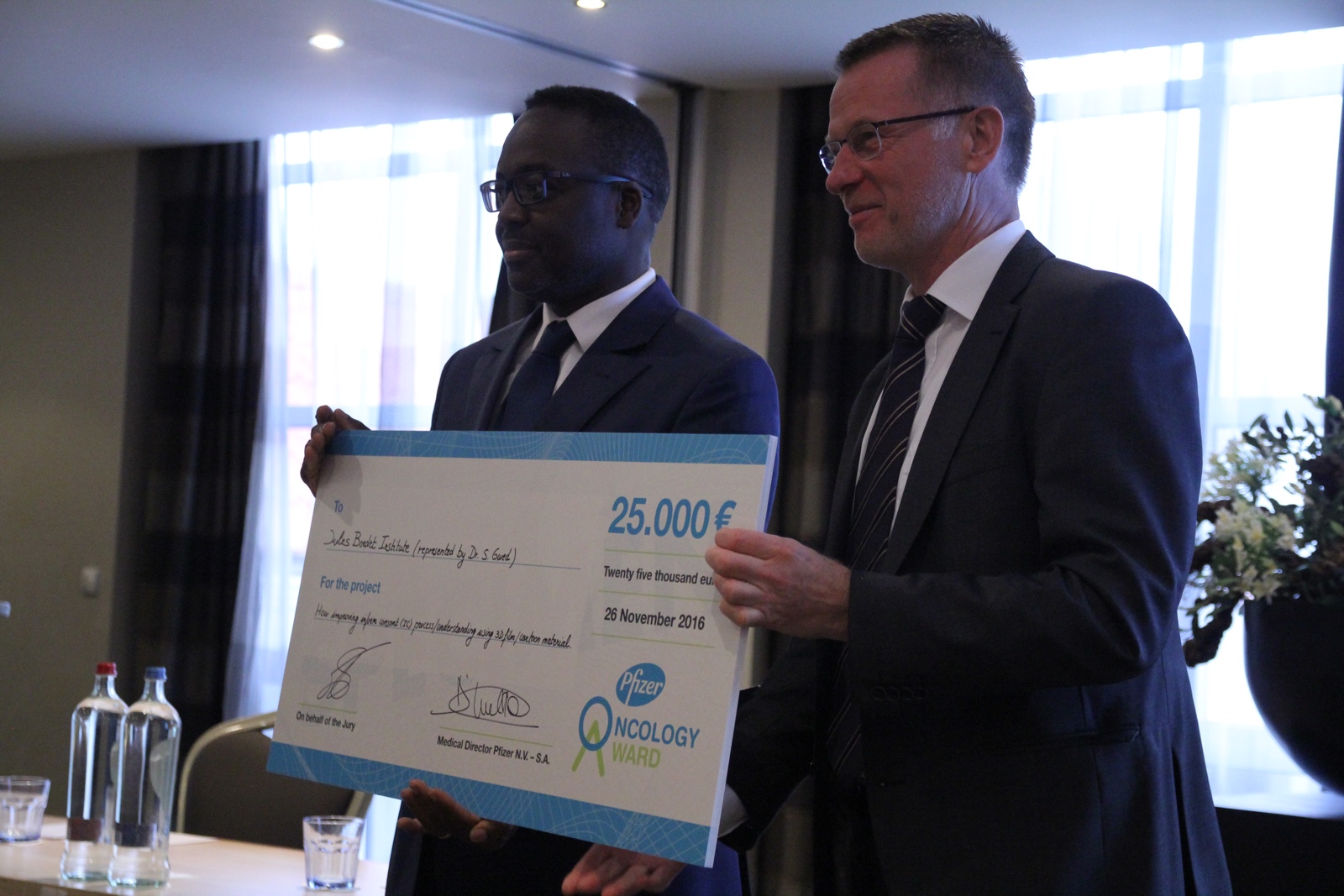
The 2016 Pfizer Oncology Award has been won by the CTCU (Clinical Trial Conduct Unit) of the Jules Bordet Institute in Brussels represented by Dr Samuel Gwed for their project: Dynamic perspective for patient information: “How improving informed consent (IC) process/understanding using 3D film or cartoon material.”
Before patients can enter a clinical trial they need to read, understand and agree with the Informed Consent Form content. This mandatory ethical and legal step often presents hurdles to many patients because it is a lengthy document which requires a lot of explanation by the staff. Its complexity can also be a reason for patients to finally not take part in a clinical trial.
Therefore, Dr Gwed explains, we propose to use multimedia as a complementary and supporting tool to the classical approach of explaining IC. We want to develop a didactic, dynamic and accessible tool which will complete the standard paper consent. The better the patient will be informed about his rights, but also about the benefits and potential risks, the better he or she will be able to decide to take part in a clinical trial.
The tool will be developed in the weeks ahead which will then be approved by an independent bioethical committee allowing for testing the tool in 2 groups of patients : one group of patients only having access to the paper version of the IC and the other group having the paper version and the didactical tool.
Picture: PRISM Prod.